Familial Hypertrophic Cardiomyopathy (FHC) affects 1 in 600 people.
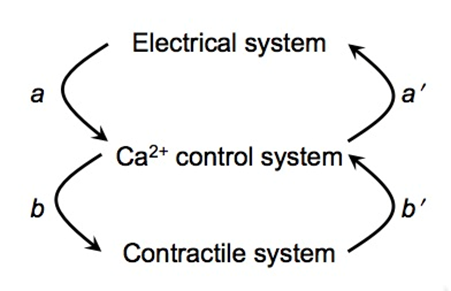 FHC is linked to mutations in contractile proteins. In some forms of FHC there is a high incidence of fatal cardiac arrhythmias (sudden cardiac death). A longstanding conundrum is how mutations in contractile proteins (affecting mechanics) cause electrical arrhythmias. We propose one possible solution to the conundrum: Mutations in the contractile proteins bring the ryanodine receptors closer together, which increases the likelihood of Ca2+ waves (Figure-1, arrow b’ ). Spontaneous Ca2+ waves, in turn, can trigger extra action potentials (Figure-1, arrow a’ ) that lead to arrhythmias.
FHC is linked to mutations in contractile proteins. In some forms of FHC there is a high incidence of fatal cardiac arrhythmias (sudden cardiac death). A longstanding conundrum is how mutations in contractile proteins (affecting mechanics) cause electrical arrhythmias. We propose one possible solution to the conundrum: Mutations in the contractile proteins bring the ryanodine receptors closer together, which increases the likelihood of Ca2+ waves (Figure-1, arrow b’ ). Spontaneous Ca2+ waves, in turn, can trigger extra action potentials (Figure-1, arrow a’ ) that lead to arrhythmias.
Mathematical modeling study on how sarcomere length affects the Ca2+ control system
We conducted modeling study to understand how mutations of contractile proteins can cause an increase in the incidence of electrical arrhythmias. The model simulations show that FHC mutations that shorten the sarcomere length, such as R92Q, increase the probability of spontaneous Ca2+ sparks and Ca2+ waves. Ca2+ waves, in turn, can trigger spontaneous (out of rhythm) action potentials that lead to arrhythmias.
The figures below show model simulations of a 3-dimensional sarcomere: the white brick-like objects are clusters of ryanodine receptors called Ca2+ release units (CRUs). Figure-A shows a model simulation with sarcomere length of 2 μm (the typical length in normal cells). A few CRUs at the edge are triggered initially, and the Ca2+ release diffuse to a few neighboring CRUs to release Ca2+. The Ca2+ release from these few CRUs dies down and nothing much happens; the system is stable. If the sarcomere length is shortened slightly to 1.8 μm, as occurs in some forms of FHC, then the Ca2+ dynamics change qualitatively as shown in Figure-B. As before, a few CRUs on the edge are triggered initially, but now the Ca2+ release from CRUS propagated to form Ca2+ waves; the system is now unstable! Figure-C shows that reducing the sarcomere length to 1.6 μm makes the system even more unstable causing the Ca2+ waves to form more quickly.
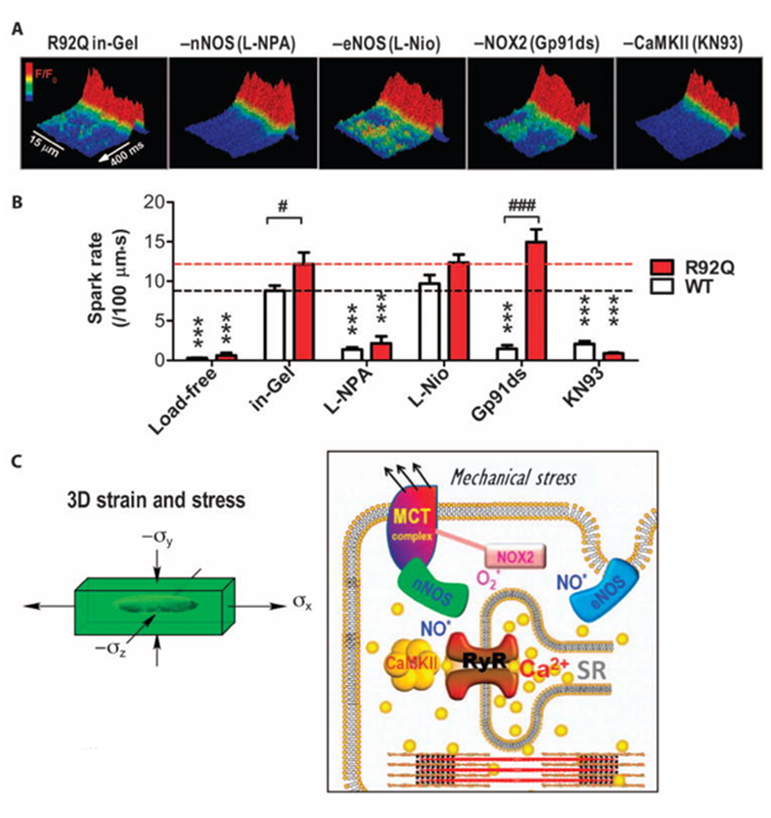
FHC shows increased sensitivity to mechanical stress
We studied a transgenic mouse model of FHC with R92Q mutation in cardiac troponin T. Using the Cell-in-Gel system to apply mechanical load on cardiac myocytes, we discovered that R92Q cells are more sensitive to mechanical stress and show more arrhythmogenic Ca2+ activities.
Relevant Publications
- Izu, L.T., S.A. Means, Y. Chen-Izu, J.N. Shadid, and C.W. Balke (2006). Interplay of Ryanodine Receptors Distribution and Calcium Dynamics. Biophys. J. 91:95—112.
- Jian Z, Han H, Zhang T, Puglisi J, Izu LT, Shaw JA, Onofiok E, Erickson JR, Chen YJ, Horvath B, Shimkunas R, Xiao W, Li Y, Pan T, Chan J, Banyasz T, Tardiff JC, Chiamvimonvat N, Bers DM, Lam KS, Chen-Izu Y*. Mechanochemotransduction During Cardiomyocyte Contraction Is Mediated by Localized Nitric Oxide Signaling. Science Signaling (2014) 317:ra27. PMID: 24643800; PMC4103414
