RESEARCH THEME
 Cardiac excitation–contraction coupling is controlled by three dynamic systems: the electrical system, the Ca2+ signaling system, and the contractile system. Recent studies by us and many other colleagues reveal that these non-linear dynamic systems interact with one other and their interactions are fundamental to heart disease mechanisms. For example, mechanical stress during muscle contraction can disrupt Ca2+ signaling to generate spontaneous Ca2+ activities, which can affect the Na+/Ca2+ exchanger and Ca2+-sensitive channels etc. to disrupt electrical activities and lead to arrhythmias. Our approach is to study integrated behavior of dynamic systems in order to understand the exact mechanism of cardiac arrhythmias. Our interdisciplinary team combines experimental investigation with mathematical modeling to conduct in-depth analyses of the dynamics systems in order to better understand heart diseases mechanisms and to develop effective therapies.
Cardiac excitation–contraction coupling is controlled by three dynamic systems: the electrical system, the Ca2+ signaling system, and the contractile system. Recent studies by us and many other colleagues reveal that these non-linear dynamic systems interact with one other and their interactions are fundamental to heart disease mechanisms. For example, mechanical stress during muscle contraction can disrupt Ca2+ signaling to generate spontaneous Ca2+ activities, which can affect the Na+/Ca2+ exchanger and Ca2+-sensitive channels etc. to disrupt electrical activities and lead to arrhythmias. Our approach is to study integrated behavior of dynamic systems in order to understand the exact mechanism of cardiac arrhythmias. Our interdisciplinary team combines experimental investigation with mathematical modeling to conduct in-depth analyses of the dynamics systems in order to better understand heart diseases mechanisms and to develop effective therapies.
RESEARCH AREAS
⇒ Cardiac Electrophysiology (more)
⇒ Ca2+ signaling system dynamics (more)
⇒ Ca2+→ electrical system interaction (more)
⇒ Mechanical analysis of cardiac myocyte contraction (more)
⇒ Mechano-chemo-transduction linking contraction → Ca2+ signaling (more)
⇒ Mathematical model of mechanical load effect on Ca2+ dynamics (…upcoming)
HEART DISEASES
Many heart diseases increase the risk of cardiac arrhythmias. We hypothesize that a common thread linking these arrhythmias is abnormal mechano-chemo-electro-transduction. Testing this hypothesis may provide a unified explanation for arrhythmias in seemingly distinct diseases and provide novel strategies for combating arrhythmias.
⇒ Hypertension induced cardiac arrhythmias and heart failure (more)
⇒ Familial Hypertrophic Cardiomyopathy (FHC, or HCM) (more)
⇒ Muscular Dystrophy Cardiomyopathy (…upcoming)
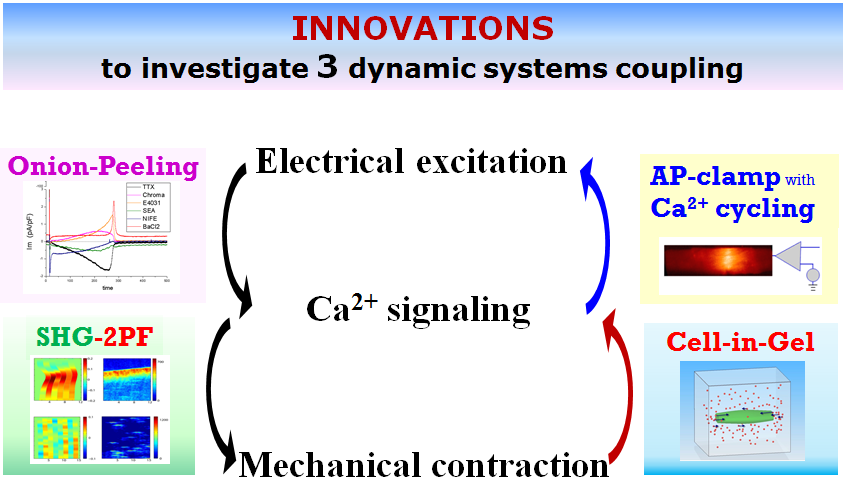
INNOVATIVE TECHNOLOGIES
|
 |
